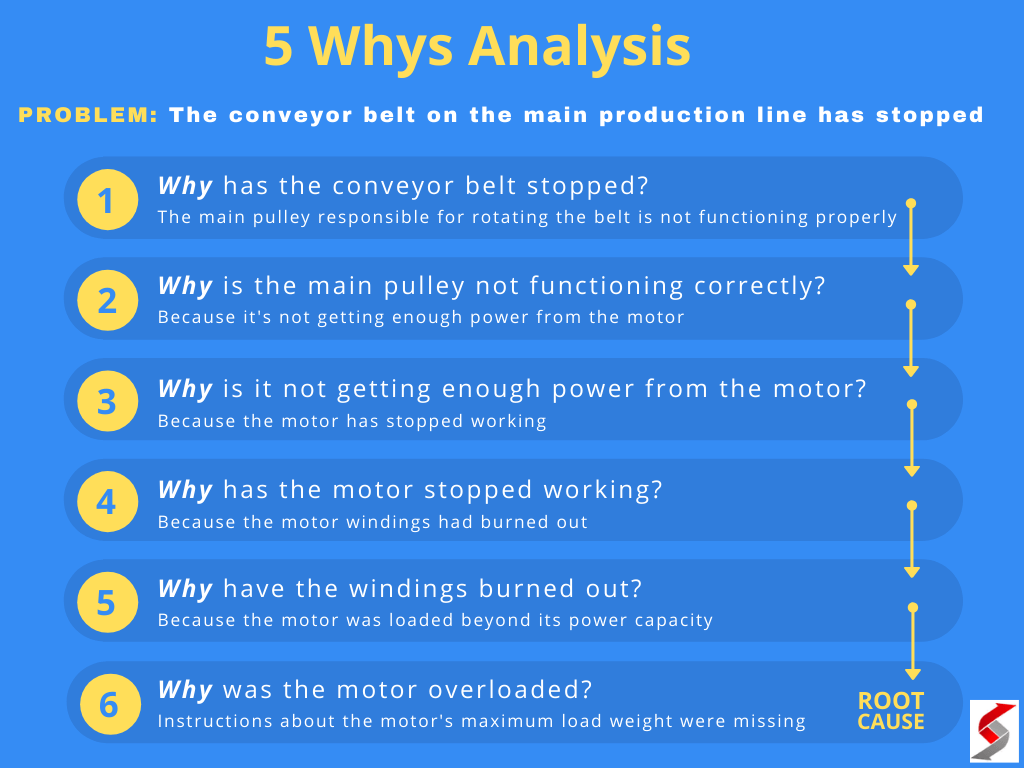
Have you ever wondered about certain historical practices, those things that just seem to stick in our collective memory, even if we don't quite grasp the "why" behind them? It's like asking why a word like "spook" became a slur during WWII, or what made "pineapple" get its name in English, when its Spanish origin was quite different. These questions, you know, they linger, making us curious about the past.
Just as we might puzzle over why "c*nt" carries such a different weight in the US compared to the UK, or why the word "pussy" came to mean a coward, the idea of lime being used in mass graves is another one of those things that sparks a lot of thought. It's a practice often depicted in stories and movies, yet its true purpose and effects are, in a way, often misunderstood by many people.
Today, we're going to explore this rather somber, yet important, historical and scientific question. We'll look at the reasons behind this practice, what lime actually does to human remains, and perhaps, what the common beliefs about it get wrong. It's about getting to the bottom of things, really, and understanding the facts.
Table of Contents
- A Look Back: The Historical Context of Lime Use
- What Exactly is "Lime" Anyway?
- Common Beliefs vs. Reality: Why People Thought Lime Was Used
- The Actual Effects of Lime on Human Remains
- Modern Approaches and Forensic Science
- Ethical Considerations and Dignity
- Frequently Asked Questions About Lime and Mass Graves
- Conclusion
A Look Back: The Historical Context of Lime Use
For a very long time, you know, people have dealt with the grim reality of mass casualties, whether from war, plague, or natural disasters. When many people die at once, it creates a huge challenge for public health and also for the proper handling of the deceased. Ancient civilizations, and then later societies, needed ways to manage large numbers of bodies, and this is where the idea of using lime, or rather, quicklime, came into play, apparently.
Historically, there was a widespread belief that quicklime could somehow purify or cleanse areas, especially those affected by disease. People thought it could stop the spread of sickness from decaying bodies. So, it was a common practice, particularly during epidemics like the Black Death, or after major battles, to throw quicklime onto bodies in common burial pits. It was, in a way, a desperate measure, driven by fear and a limited understanding of disease transmission, you know, back then.
The use of quicklime in these situations wasn't always based on solid scientific evidence, but rather on observation and, arguably, a bit of hope. They saw a reaction when quicklime met moisture, and perhaps this visible activity led them to believe it was doing something powerful to the bodies. It's a bit like how people once thought certain remedies worked, just because they saw a strong reaction, without truly grasping the underlying chemistry, you know, the real facts.
What Exactly is "Lime" Anyway?
When we talk about "lime" in this context, we're usually not talking about the fruit, of course! It's actually a chemical compound derived from limestone. There are, however, a couple of forms of lime that are important to distinguish, because they behave quite differently when they come into contact with organic matter, and, you know, that really matters here.
Quicklime (Calcium Oxide)
Quicklime, which is chemically known as calcium oxide (CaO), is made by heating limestone (calcium carbonate) to very high temperatures. This process, called calcination, drives off carbon dioxide, leaving behind a highly reactive white solid. Quicklime is, basically, incredibly absorbent and reacts very strongly with water, releasing a lot of heat in the process. This reaction is called slaking, and it